We use DNA to create a new world of structural engineering and biological computation
News
March 1, 2024
Pallav Kosuri named Hearst Foundation Developmental Chair!
February 12, 2024
Ryan Fantasia and Lauren Takiguchi present their work to a curious audience at Biophysical Society Annual Meeting in Philadelphia!
January 17, 2024
Kosuri Lab & Gage Lab win $1.3M award from W.M. Keck Foundation!
January 11, 2024
News article in The Scientist describing our work with Mark Rober.
December 24, 2023
Happy holidays from all of us at the Kosuri Lab!
December 19, 2023
Congratulations Ph.D. student Ryan Fantasia on winning an SRF Career Advancement Award!
November 1, 2023
News article on Freethink.com discussing our work with Mark Rober.
September 30, 2023
Collaboration with @MarkRober: We used DNA to make the world’s smallest NERF blaster!
[Watch YouTube video] (Update: 35M+ views as of Jan 2024!)
Using DNA origami, we made a molecular-scale replica of a NERF blaster, 3 million times smaller than the original.
For the full story, watch the NanoNERF video on Mark Rober’s YouTube channel.
For a technical description, read the NanoNERF manuscript on bioRxiv by Takiguchi et al.
September 26, 2023
Lauren Takiguchi starts her PhD in Biophysics at Stanford University – you’ve done amazing work in the lab and we wish you all the best on your next adventure!
September 14, 2023
Amanda Wacker is on a streak! Congratulations on being awarded the Salk Women & Science Professional Development Award!
July 28, 2023
Congratulations Amanda Wacker on winning the Dan and Martina Lewis Biophotonics Fellowship!
May 22, 2023
Congratulations Yuening Liu on winning the Edwards-Yeckel Postdoc Professional Development Award!
March 31, 2023
Congratulations to Jocelyn Olvera on being awarded an NSF Graduate Research Fellowship (GRFP)!
Congratulations also to Jerry Wu on winning a Goldwater Scholarship!
February 22, 2023
Kosuri Lab goes to Biophysical Society Annual Meeting (BPS 2023)!
February 12, 2023
Welcome to the lab Julia Rune!
Research
The Kosuri Lab studies how movement gives rise to biological function across scales, from molecular motors to muscles.
Molecular scale
What are the physical movements during the genetic processes of transcription, chromatin remodeling, and gene editing?
Ensemble scale
How do contributions from single molecules add up in an ensemble to produce collective mechanical behavior?
Organ scale
How does the mechanical and cellular architecture of the heart contribute to the pathophysiology of heart disease?
We are addressing these questions in three tracks, taking a cross-disciplinary approach that makes use of molecular design, computational methods, and functional imaging. When needed, we invent new technology.
Track 1: Using self-assembled DNA devices to study molecular movements
Genetic processes such as transcription, replication, DNA repair and editing are composed of a series of mechanical movements. Most of these movements have remained challenging or impossible to measure.
To see these movements, we make use of the rapidly advancing field of DNA self-assembly, and in particular the DNA origami method, which enables design and assembly of custom 3D structures. Using DNA origami, we are developing a suite of new devices that enable direct measurement of molecular movements.
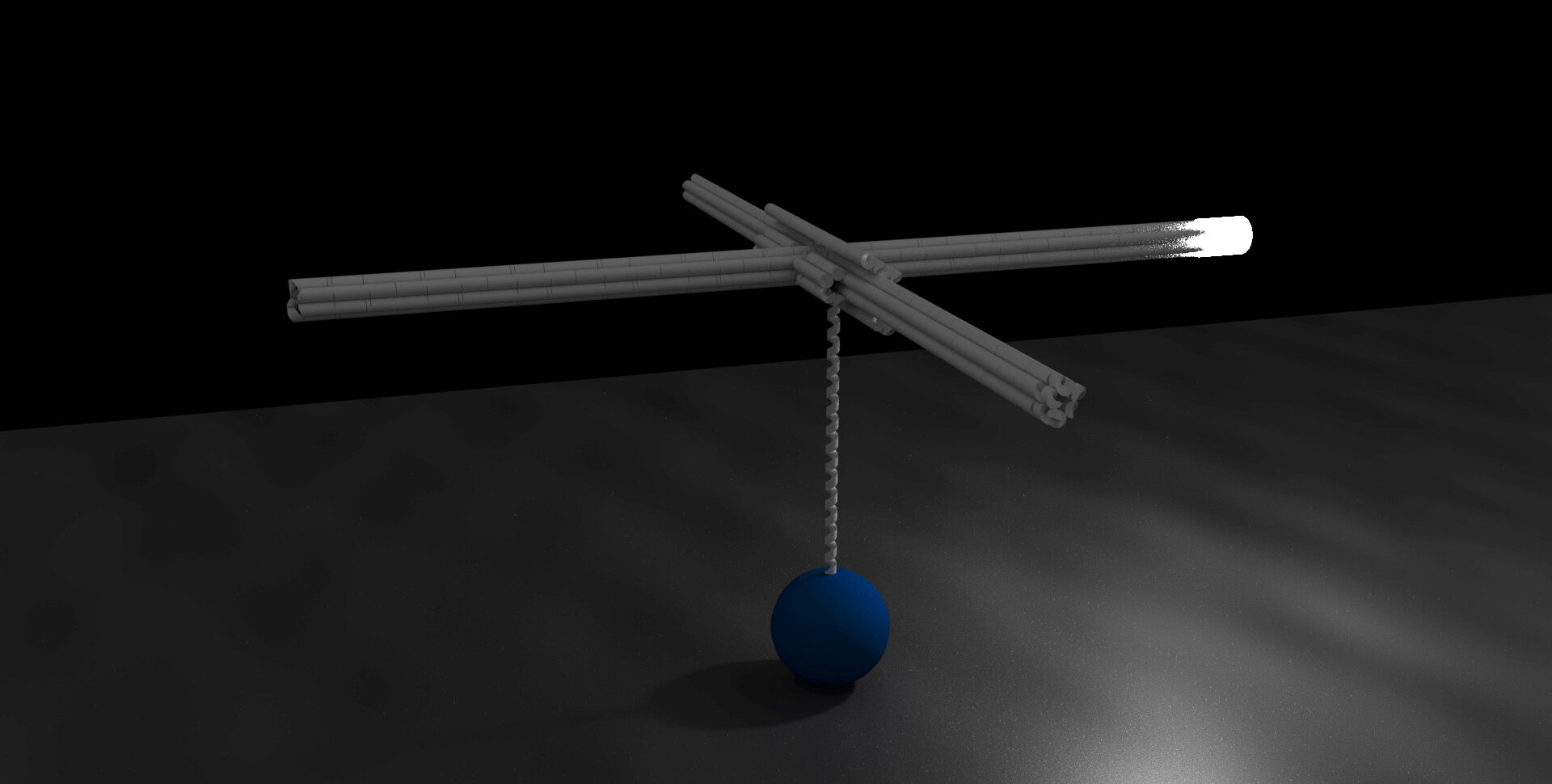
Our first such technology, Origami Rotor Based Imaging and Tracking (ORBIT), uses self-assembled DNA origami rotors to amplify and visualize molecular rotation.
ORBIT uses DNA rotors to visualize transcription by RNA polymerase
DNA origami rotors amplify the rotational movement generated by RNA polymerase (blue) as it transcribes DNA (vertical helix). A rotor blade tip has been labeled with a fluorescent marker (white).
Raw microscope data used to track the fluorescent tip of a rotor blade (trajectory overlaid in white).
RNA polymerase appears to rotate DNA in distinct ~35 degree steps, potentially reflecting the helical twist between DNA base pairs.


We record the movements of thousands of molecules in each experiment, revealing a rich diversity of activities.

Our DNA origami approach makes it possible to launch a new kind of investigations into the mechanisms of DNA interacting proteins. We are excited to see how our concept of using DNA origami devices can be further put to use to address some of the most fundamental questions in molecular biology and genetics.
Future projects in Track 1 include:
Using ORBIT to investigate transcriptional stochasticity and regulation.
Using ORBIT to investigate how chromatin remodeling enzymes move nucleosomes on DNA.
Creating a DNA origami based single-molecule assay to study gene editing enzymes.
Developing a high-throughput DNA origami based assay to detect small molecule-DNA binding.
Multiplexing DNA origami based assays for applications in drug screening.

Track 2: Synthetic muscle as a model system for contractile tissues
How do the individual contributions of molecular components add up to produce the mechanical behavior of a muscle?
Answering this question will require a quantitative theory wherein the tissue’s mechanics can be described in terms of its molecular components. With a bottom-up description of muscle, we would be able to better predict pathological outcomes resulting from mutations, stress or damage, as well as the effect of therapeutic interventions.
To address this challenge, we are designing a model system that reproduces the basic features of muscle and that affords exquisite control of the geometry and stoichiometry of its components. This synthetic muscle experimental system will allow us to measure scaling phenomena and test theoretical and computational models, laying the groundwork for a bottom-up quantitative description of muscle.

Track 3: Creating a mechanical atlas of the heart
How does mechanical function and dysfunction of the heart arise from the tissue's cellular and molecular organization?
We are using novel imaging methods including spatially resolved transcriptomics to create functional 3D maps of the heart. These maps will help us study how the molecular and cellular structure of the heart leads to its mechanical function or dysfunction. In particular, we are focusing on tissue remodeling and fibrosis, two hallmarks of heart failure, to build quantitative models of disease progression and to identify new therapeutic strategies.
People
Pallav Kosuri, Ph.D.
Assistant Professor
I studied physics in college at KTH in Stockholm, Sweden (where I’m from). As a PhD student in the lab of Julio Fernandez at Columbia University, I studied the mechanics of protein folding and discovered that we can modulate this process to alter the elasticity of muscles. I then moved to Harvard University as a postdoc in the lab of Xiaowei Zhuang, where I used DNA to invent and build nanosensors that make it possible to see molecular movements. Since starting my lab at the Salk Institute in 2021, I have been excited about building a diverse and thriving research community, while training future leaders in biophysics. When I’m not in the lab, you can find me rock climbing, skiing, or learning to surf.
pkosuri [at] salk.edu
Salk faculty webpage
[CV]
Roopal Rawani
Research Lab Administrator
I am a friendly team player who joined Salk in November 2019. As a graduate from CUNY college in Finance, I bring forth high quality management, organizational skills and have a self-motivated drive to achieve excellence. Since graduation, I have had a chance to work in many diverse fields, working at - anywhere from a small marketing company to the non-profit to the major university – I have done it all. I have found Salk to be the place where my contributions will be most valuable - I am an administrator for four labs. I am excited to be a part of the institution that has made major discoveries and continues to do so. When not at work, I love spending time outdoors – strolling around farmers markets (they have the best samples! and a spicy mango drink!), Padres games, hiking, long drives (everything is far in CA - says a native New Yorker) and exploring new coffee shops with my family.
Yuening Liu, Ph.D.
Postdoctoral fellow
I received my B.S. in Pharmaceutical Sciences from University of Toledo and joined the College of Pharmacy and Pharmaceutical Sciences at Washington State University as a graduate student 2016. During my Ph.D. training, I studied cardiovascular disease related cell death and investigated signaling pathways involved in cardiomyocyte hypertrophy, necrosis and myocardial ischemia/reperfusion injury. I am very excited to join Dr. Kosuri’s lab, where I will explore new research directions including using spatially resolved transcriptomics to characterize signaling pathways involved in heart failure. With my research, I would like to shed new light on cardiovascular disease initiation and progression, providing insights for better diagnosis and treatment. When I’m not in the lab, you can find me reading, hiking, and exploring SD.
Brian Tenner, Ph.D.
Postdoctoral fellow
I completed my PhD in Molecular Biophysics from Johns Hopkins University in 2019, where I engineered fluorescent protein-based biosensors to monitor cell signaling events. Since then, I’ve held innovative roles in the biotechnology space, leading assay development and creating scalable assays for proteomics and sequencing-based applications. In Summer 2024, I joined Dr. Pallav Kosuri’s lab, where I am using DNA origami and rotational tracking to study enzymatic dynamics at a single-molecule resolution. My work combines advanced molecular engineering, data-driven computational modeling, and cutting-edge high-throughput methodologies to enhance the precision and scalability of DNA assays. Outside the lab, I enjoy exploring nature, visiting airplane museums, playing trivia, and staying up-to-date with scientific discoveries.
Amanda Wacker
Ph.D. student
I am a PhD student and NIH T32 trainee at UC San Diego. My current research is focused on discovering how DNA-interacting molecules work, by using DNA origami and single molecule microscopy techniques. I completed my undergraduate degree at Florida State University in Biology, and my research interests brought me to spend some summers as an intern at Harvard Medical School and the NASA Ames Research Center. I am also passionate about science communication and outreach. I created and now co-host the Triplicates Podcast. This podcast aims to provide advice, support, and resources to students, especially first-generation and minority students, as they navigate higher education programs. To help build a stronger and more inclusive community, I helped establish the graduate chapter of Out in STEM at UC San Diego, run a LGBTQ+ centered book club for grad students, and work as a community assistant at Graduate and Family Housing. Outside of the lab, I can be found scuba diving (always on the lookout for octopuses) and reading a book that wasn’t on my TBR.
Ryan Fantasia
Ph.D. student
I am a Ph.D. student in the Biological Sciences Department at the University of California San Diego. My focus is on engineering nucleic-acid-based tools to quantify molecular interactions. I am motivated by an overarching interest in Quantitative Biology and Biophysics. I graduated with a Bachelor of Science in Mathematics and a Minor in Biology from the University of Massachusetts Amherst in 2018. Following college, I spent three years as a research technician in the department of anesthesia at Massachusetts General Hospital. Outside of the lab I enjoy exploring the west coast, trying new sports, reading at the beach and learning to cook new recipes.
Delisa Ramos
Ph.D. student
I am a PhD student in the Biological Sciences program at UC San Diego. My current research focuses on using single molecule imaging techniques to investigate local and global mechanisms of aging in the heart and the brain. I received a B.S. in Genetics from UT Austin and started my research career in the Taylor lab structurally characterizing novel bacterial defense systems. Outside of lab you can generally catch me on a run around Mission Bay, basking in the sun with a book, or trying out local restaurants with friends.
Deevanshu Goyal
Computational Research Assistant II
I graduated from the Machine Learning and Data Science Master’s program at UCSD in 2024 and joined Prof. Kosuri’s lab soon after. I am interested in computational biology with a particular passion in applying statistical modeling and machine learning approaches for bio-computational applications. In the past, at IIT Delhi I have worked on analysing RNA-seq reads of exosome cultures isolated from lung cancer patients to identify biomarkers for liquid biopsy purposes. While interning at Sarepta Therapeutics, I trained ML models on CRISPR-generated datasets for insights into candidate selection for gene therapy treatments. I am very excited to join the Kosuri Lab where I will continue expanding my current skillset and knowledge base by conducting analysis of spatial transcriptomic datasets for various downstream applications. Outside of lab, you will find me watching anime, reading fiction, and consuming pop culture in any form possible.
Annabelle Coles
Computational Lab Technician I
I am an undergraduate student at UCSD studying Biology with a specialization in Bioinformatics and a minor in Computer Science. Over the past two years, I’ve been learning how to code and consequently discovered my passion for computational biology. As a student, I am excited to learn and apply modern methods of computation to further understand biological mechanisms. Before joining the Kosuri Lab, I was an undergraduate researcher at the UCSD School of Medicine and a research intern at the Qualcomm Institute; through these research opportunities, I developed and refined my biological and computational research skills. Outside of school, you can find me hiking, stargazing, and singing karaoke.
Boyu Barry Liu
Research Assistant I
I recently graduated from UCSD studying Molecular and Cell Biology and I am interested in the world inside cells. Previous research experiences with microbial genes and proteins intrigued me to look at these molecules in more detail at a minuscular level. At the Kosuri lab, I am diving into the world of nanobiology using optical light microscopy to the study nucleic acids and their interactions with different biological molecules. Outside of the lab, you can find me cycling, playing tennis, and skiing.
Nicholas Monell
Undergraduate student
I am an undergraduate student at UCSD studying Bioengineering: Bioinformatics with a passion for microscopy and image analysis. Through previous research within the UCSD Center for Epigenomics, I cultivated computational techniques that I use in conjunction with computer science to interpret elegant biological processes. In the Kosuri Lab, I have the pleasure of utilizing methods that will allow me to apply my experience in further understanding the movement of DNA and other nanomolecules. Outside of school, you’ll find me doing archery, writing poems, or learning a new fun fact!
Jonah Castiglione
Undergraduate student
I am an undergraduate student at UCSD studying Computer Science, with an emphasis on Machine Learning in healthcare and biological research. My previous research at the Nezafat Translational Cardiovascular Imaging Lab at Beth Israel Hospital allowed me to develop clinical ML tools for identifying disease on a macroscopic level, and now I hope to do the same, but on a microscopic level at the Kosuri Lab. Through my current work, I aim to apply ML to gene expression analysis to help advance our understanding of how our heart express themselves through health and sickness.
Alumni
Jerry Wu
Undergraduate student
Current position: PhD student in Bioengineering at Princeton University.
Lauren Takiguchi
Research Assistant
Current position: PhD student in Biophysics at Stanford University.
Rebecca Christensen
Research Assistant
Current position: PhD student in Microbiology at Columbia University
Julia Rune
Visiting Master’s student
Current position: PhD student in Theoretical Chemistry at KTH Royal Institute of Technology, Stockholm, Sweden.
Abigail Neininger-Castro, Ph.D.
Postdoctoral fellow
Current position: Application Development Data Scientist at ONI
Claire Ricketts
Undergraduate student
Current position: Research Engineer at Ansa Bio
We are always looking for highly motivated postdoctoral fellows, graduate students and research assistants!
Philosophy

Our mission is to seek knowledge for the benefit of humanity, to help solve our world’s most pressing problems, and to inspire people to think in new ways about what is possible to achieve.
As scientists, we must strive to be the best version of ourselves, and set our internal standards for ethics and integrity higher than what is expected from us. We must seek to build an inclusive community where we welcome diversity of background and encourage diversity of thought. The responsibility also rests on us to promote these values in society at large.
We need to be confident in our abilities yet critical of our practice. In our research, we must seek to disprove our own assumptions and hypotheses. And as mentors, we must continuously refine our approach so as to best support our mentees, and always stay open to feedback.
Expectations of postdocs
We expect that you will seek to realize your potential as an independent scientist. You should be goal-driven, critical and inquisitive, unafraid of problem solving, and supportive of your team. As a member of our lab, you will have opportunities to train in all the aspects that will make you impactful in science – academic or otherwise – including project design, writing, giving talks, and mentoring. You should communicate clearly what you need to be successful, and we will check in on a regular basis to make sure that we stay aligned with your goals.
Expectations of graduate students
We expect you to be curious, dedicated, excited to create new science, and open to change your mind when your experiments prove you wrong. You will have your own project, for which you will be involved in every aspect, from research design to writing a paper, and you are expected to be the lead author on the resulting publications. You will have the same access to career-building opportunities as the postdocs, and we will expect you to broaden your expertise through conference presentations, collaborations, grant writing, and potentially technology transfer and patenting. We expect you to ask lots of questions, communicate your concerns, and ask for help or guidance when you need it. We are all here to support you in your academic journey, and we will do our best to ensure that it is an exceptional one.
Expectations of PI
You can expect me to support you at every stage in your career, from the moment you join the lab to the time when you are preparing for your next job, and beyond. I will listen to you, take your feedback seriously, and strive to be available whenever you need advice or guidance. Most importantly, I will care about your physical and mental wellbeing, your personal development, and your success as a scientist.
Publications
Selected publications
DNA origami rotors
Rotation tracking of genome-processing enzymes using DNA origami rotors
Kosuri P*, Altheimer BD*, Dai M, Yin P, Zhuang X (*co-first authors)
Nature (2019)
Protein folding
Protein folding drives disulfide formation
Kosuri P, Alegre-Cebollada J, Feng J, Kaplan A, Ingles-Prieto A, Badilla C, Stockwell BR, Sanchez-Ruiz JM, Holmgren A, Fernandez JM
Cell (2012)
Muscle elasticity
S-glutathionylation of cryptic cysteines enhances titin elasticity by blocking protein folding
Alegre-Cebollada J*, Kosuri P*, Giganti D, Eckels E, Rivas-Pardo JA, Hamdani N, Warren CM, Solaro RJ, Linke WA, Fernandez JM (*co-first authors)
Cell (2014) cover story
Muscle contraction
Work done by titin protein folding assists muscle contraction
Rivas-Pardo JA, Eckels EC, Popa I, Kosuri P, Linke WA, Fernandez JM
Cell Reports (2016)
Full list of publications
Dye-cycling DNA origami rotors for long-term tracking of transcription at base-pair resolution
Wacker A, Fantasia RJ, Tenner BJ, Wu J, Monell NA, Kosuri P
bioRxiv (2026)
Measuring nanoscale torques with cylindrical-polarization-based interferometric scattering microscopy
Vala M, Kopal I, Takiguchi L, Shaidiuk Y, Lužný V, Bujak Ł, Kosuri P, Piliarik M
arXiv (2026)
ImmGenMaps, an open-source cartography of the immune system
Reina-Campos M, Farhi SL, Benoist C, ImmGenMaps Group
Nature Immunology (2025) Correspondence
Single-molecule DNA dynamics with graphene energy transfer
Kosuri P
Nature Methods (2025) News & Views
Tetra-gel enables superior accuracy in combined super-resolution imaging and expansion microscopy
Lee H, Yu CC, Boyden ES, Zhuang X, Kosuri P
Scientific Reports (2021)
Rotation tracking of genome-processing enzymes using DNA origami rotors
Kosuri P*, Altheimer BD*, Dai M, Yin P, Zhuang X (*co-first authors)
Nature (2019)
Work done by titin protein folding assists muscle contraction
Rivas-Pardo JA, Eckels EC, Popa I, Kosuri P, Linke WA, Fernandez JM
Cell Reports (2016)
Predicting readmission of heart failure patients using automated follow-up calls
Inouye S, Bouras V, Shouldis E, Johnstone A, Silverzweig Z, Kosuri P* (corresponding author)
BMC Medical Informatics and Decision Making (2015)
S-glutathionylation of cryptic cysteines enhances titin elasticity by blocking protein folding
Alegre-Cebollada J*, Kosuri P*, Giganti D, Eckels E, Rivas-Pardo JA, Hamdani N, Warren CM, Solaro RJ, Linke WA, Fernandez JM (*co-first authors)
Cell (2014) cover story
Picomolar amyloid-β peptides enhance spontaneous astrocyte calcium transients
Lee L, Kosuri P, Arancio O
Journal of Alzheimer's Disease (2014)
Force dependency of biochemical reactions measured by single-molecule force-clamp spectroscopy
Popa I*, Kosuri P*, Alegre-Cebollada J, Garcia-Manyes S, Fernandez JM (*co-first authors)
Nature Protocols (2013)
Protein folding drives disulfide formation
Kosuri P, Alegre-Cebollada J, Feng J, Kaplan A, Ingles-Prieto A, Badilla C, Stockwell BR, Sanchez-Ruiz JM, Holmgren A, Fernandez JM
Cell (2012)
Direct observation of disulfide isomerization in a single protein
Alegre-Cebollada J, Kosuri P, Rivas-Pardo JA, Fernandez JM
Nature Chemistry (2011)
Protease power strokes force proteins to unfold
Alegre-Cebollada J, Kosuri P, Fernandez JM
Cell (2011) preview
Single-molecule paleoenzymology probes the chemistry of resurrected enzymes
Perez-Jimenez R, Ingles-Prieto A, Zhao Z, Sanchez-Romero I, Alegre-Cebollada J, Kosuri P, Garcia-Manyes S, Kappock TJ, Tanokura M, Holmgren A, Sanchez-Ruiz JM, Gaucher EA, Fernandez JM
Nature Structural & Molecular Biology (2011)
Single-molecule force spectroscopy approach to enzymatic catalysis
Alegre-Cebollada J, Perez-Jimenez R, Kosuri P, Fernandez JM
Journal of Biological Chemistry (2010)
Kalman filter estimates of the contour length of an unfolding protein in single-molecule force spectroscopy experiments
Fernandez VI, Kosuri P, Parot P, Fernandez JM
Review of Scientific Instruments (2009)
Partially folded equilibrium intermediate of the villin headpiece HP67 defined by 13C relaxation dispersion
O’Connell NE, Grey MJ, Tang Y, Kosuri P, Miloushev VZ, Raleigh DP, Palmer AG
Journal of Biomolecular NMR (2009)
Diversity of chemical mechanisms in thioredoxin catalysis revealed by single-molecule force spectroscopy
Perez-Jimenez R, Li J, Kosuri P, Berne BJ, Fernandez JM
Nature Structural & Molecular Biology (2009)
Force-clamp spectroscopy detects residue co-evolution in enzyme catalysis
Perez-Jimenez R, Wiita AP, Rodriguez-Larrea D, Kosuri P, Gavira JA, Sanchez-Ruiz JM, Fernandez JM
Journal of Biological Chemistry (2008)
Coupling of ribosomal L1 stalk and tRNA dynamics during translation elongation
Fei J, Kosuri P, MacDougall DD, Gonzalez RL
Molecular Cell (2008)
Development of a RILIS ionisation scheme for gold at ISOLDE, CERN
Marsh BA, Fedosseev VN, Kosuri P
Hyperfine Interactions (2006)
Contact information
Kosuri Lab
Salk Institute for Biological Studies
10010 N Torrey Pines Rd
La Jolla, CA 92037
pkosuri [at] salk.edu

Join the team!
We are always interested in applications from passionate people from all backgrounds who are willing to work hard to create the next generation of science and technology!
We currently recruiting postdoctoral fellows, graduate students, and computational scientists.
For UCSD graduate students, we consider rotation students from across all programs and backgrounds.
If you are interested in joining our team, send us an email at pkosuri[at]salk.edu!









































